Introduction: Hemophilia A is an X-linked ( F8 gene) recessive disorder of hemostasis that results in insufficient factor VIII (FVIII) activity. Adeno-associated virus (AAV)-based gene therapy enables delivery of a modified F8 cDNA, allowing synthesis of functional endogenous FVIII, which prevents bleeding events. We present updated results with nearly 4 years of follow-up on an ongoing gene therapy study in participants with severe hemophilia A (FVIII activity <1%).
Methods:The phase 1/2 Alta study (NCT03061201) is a dose-ranging study of giroctocogene fitelparvovec (PF-07055480, previously called SB-525), a recombinant AAV serotype 6 vector encoding a modified B-domain-deleted F8 coding sequence. Four ascending doses of giroctocogene fitelparvovec (9e11, 2e12, 1e13, and 3e13 vg/kg) were infused into adults aged ≥18 years with severe hemophilia A across 4 cohorts (n=2 each). The high-dose (3e13 vg/kg) cohort was expanded to 5 participants. Key endpoints included safety, circulating FVIII activity, use of FVIII replacement therapy, and frequency of bleeding events.
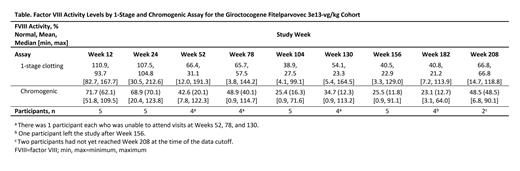
Results: Eleven male participants were enrolled in the study (mean [SD] age, 30.3 [7.8] years; White, 81.8%). As of the cutoff date (May 19, 2023), participants had been followed for 153 to 290 weeks. Two participants left the study after Week 156. Of the remaining, 1 participant had not yet completed 4 years (208 weeks). The most common treatment-related adverse events (AEs) reported in the high-dose cohort (n=5) were elevated liver enzymes and infusion-related reactions: increased alanine aminotransferase (ALT; n=3 [60.0%]), increased aspartate aminotransferase (AST; n=2 [40.0%]), pyrexia (n=3 [60.0%]), and tachycardia (n=2 [40.0%]). Treatment-related serious AEs were reported in 1 participant in the high-dose cohort who experienced hypotension and fever, with onset ≈6 h after infusion; the events fully resolved with treatment. AEs (all causality) of ALT increases requiring ≥7 days of corticosteroids were observed in 4 of 5 participants in the high-dose cohort. ALT elevations were managed with tapering courses of corticosteroids (median duration: 56 days; range: 7-135 days), with maintenance of efficacious levels of FVIII activity. Participants in the high-dose cohort have not required steroids since Week 65, have had ALT values in the normal range (follow-up: 156-208 weeks) and normal findings via liver MRI (follow-up: 104-208 weeks). No participant developed a confirmed inhibitor to FVIII. No thrombotic events or liver masses have been detected. Of the 5 participants in the high-dose cohort, 2 had data available through Week 208 and FVIII activity was maintained in the mild to normal range ( Table), consistent with Week 156 results. Of those without Week 208 data, 2 had data through Week 182. One participant maintained FVIII activity in the mild range (14.1% and 24.1% of normal, measured with a chromogenic and 1-stage assay, respectively); the other had FVIII activity of 3.1% and 7.2%. The remaining participant left the study after Week 156, with FVIII activity maintained in the mild range (11.8% and 22.9%). In the high-dose cohort, the mean annualized total bleeding rate [(number of all bleeding episodes starting 3 weeks after study drug infusion) / (observation period in years)] was 0 for the first year post infusion and 1.2 (SD 2.58) throughout the total duration of follow-up. In this cohort, the participant with the lowest FVIII activity level experienced a total of 22 bleeds, with 21 necessitating treatment (8 traumatic; 7 spontaneous; 6 unknown). The other 4 participants had no or very minimal bleeds, including 1 who experienced a bleed in a target joint. No participants in the high-dose cohort have resumed prophylaxis.
Conclusion:A single infusion of giroctocogene fitelparvovec gene therapy in participants with severe hemophilia A remains generally well tolerated over a period of nearly 4 years post infusion, with associated increases in FVIII levels in the moderate to normal range, without sustained AEs and with no AEs associated with increased liver function tests since Week 59. The ongoing phase 3 study (NCT04370054) in a larger cohort will provide more long-term data on the safety and durability of giroctocogene fitelparvovec in participants with moderately severe to severe hemophilia A.
OffLabel Disclosure:
Giermasz:Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees; BioMarin Pharmaceutical Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants; uniQure: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi Genzyme: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bioverativ, Genentech/Roche, Biomarin, uniQure, American Thrombosis and Hemostasis Network: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Visweshwar:Biogen Idec: Consultancy. Leavitt:BioMarin, Pfizer: Research Funding; Catalyst BioSciences: Research Funding; BioMarin: Consultancy; Catalyst Biosciences: Consultancy; Genetech, HEMA Biologics: Consultancy; Merck: Consultancy. Konkle:Pfizer: Research Funding; Spark: Research Funding; Takeda: Research Funding; uniQure: Research Funding; Pfizer: Consultancy; Sigilon: Consultancy; Spark: Consultancy; Octapharma: Consultancy; Regeneron: Consultancy; BioMarin: Membership on an entity's Board of Directors or advisory committees. Rupon:Pfizer: Current Employment, Current equity holder in publicly-traded company. Dirusso:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Tseng:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. de los Angeles Resa:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Ganne:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Agathon:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Plonski:Pfizer: Consultancy, Current equity holder in publicly-traded company. Rouy:Sangamo Therapeutics: Current Employment, Divested equity in a private or publicly-traded company in the past 24 months. Cockroft:Sangamo Therapeutics: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Fang:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Arkin:Pfizer: Current Employment, Current equity holder in publicly-traded company.
Hemophilia A, a sex-linked (F8 gene) disorder of hemostasis,results in insuffi cient factor VIII (FVIII) activity. Adeno-associatedvirus (AAV) mediated gene transfer enables the delivery of amodifi ed functional F8 coding sequence to hepatocytes. Thissubsequently synthesizes FVIII at levels preventing unprovokedbleeding events in the absence of exogenous FVIII.Giroctocogene fi telparvovec (PF-07055480, previously called SB-525) is a recombinant AAV serotype 6 vector encoding amodifi ed B-domain deleted F8 coding sequence. Giroctocogenefi telparvovec is currently under investigation in a phase 1/2study for the treatment of severe (factor VIII activity <1%)hemophilia A and a phase 3 study for the treatment ofmoderately severe to severe (factor VIII activity <=1%)hemophilia A.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal